Hi Friends .
Like Shear and Comments these Post.
The word acid originates from the Latin words acidus or acere, which signify "harsh," since one of the attributes of acids in water is an acrid taste (e.g., vinegar or lemon juice).
This table offers a review of the key properties of acids contrasted and bases.
Rundown of Acid and Base Properties :-
Property Acid Base
pH less than 7 greater than 7
taste sour bitter or lathery (e.gheati pop)
(e.g.vinegar)
scent burning sensation often no smell (special case is alkali)
surface sticky slippery
reactivity reacts with metals reacts with a few fats and oils
to deliver hydrogen gas
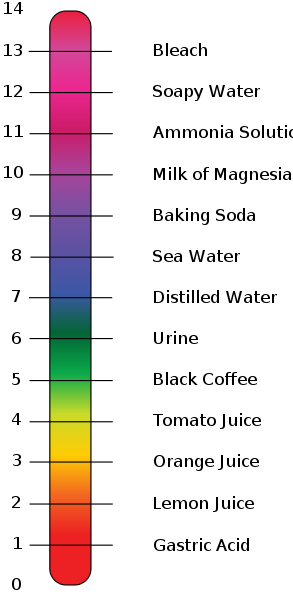 Acid Examples :-
Acid Examples :-
Arrhenius, Brønsted-Lowry, and Lewis Acids :-
There are various methods for characterizing acids. An individual alluding to "an acid" is typically alluding to an Arrhenius or Brønsted-Lowry acid. A Lewis acid is commonly called a "Lewis acid." The purpose behind the varying definitions is that these various acids do exclude a similar arrangement of atoms:
Arrhenius Acid: By this definition, an acid is a substance that builds the convergence of hydronium particles (H3O+) when added to water. You may likewise think about expanding the convergence of hydrogen particle (H+) as another option.
Brønsted-Lowry Acid: By this definition, an acid is a material equipped for going about as a proton benefactor. This is a less prohibitive definition since solvents other than water are not barred. Basically, any intensify that can be deprotonated is a Brønsted-Lowry acid, including normal acids, in addition to amines, and liquor. This is the most generally utilized definition of an acid.
Lewis Acid: A Lewis acid is an intensify that can acknowledge an electron pair to shape a covalent bond. By this definition, a few aggravates that don't contain hydrogen qualify as acids, including aluminum trichloride and boron trifluoride.
These are instances of kinds of acids and explicit acids:
Arrhenius acid
Monoprotic acid
Lewis acid
Hydrochloric acid
Sulfuric acid
Hydrofluoric acid
Acidic acid
Stomach acid (which contains hydrochloric acid)
Vinegar (which contains acidic acid)
Citrus extract (found in citrus natural products)
Solid and Weak Acids :-
Acids might be distinguished as either solid or feeble dependent on how totally they separate into their particles in water. A solid acid, for example, hydrochloric acid, totally separates into its particles in water. A feeble acid just somewhat separates into its particles, so the solution contains water, particles, and the acid (e.g., acidic acid).
Acid: Acid definition,types and properties.
Acid and Acid Properties
Acid,
any substance that in water solution tastes harsh, changes the shade of
specific markers (e.g., blushes blue litmus paper), responds with
certain metals (e.g., iron) to free hydrogen, responds with bases to
frame salts, and advances certain concoction responses (acid catalysis).
Instances of acids incorporate the inorganic substances known as the
mineral acids—sulfuric, nitric, hydrochloric, and phosphoric acids—and
the natural mixes having a place with the carboxylic acid, sulfonic
acid, and phenol gatherings. Such substances contain at least one
hydrogen iotas that, in solution, are discharged as emphatically charged
hydrogen particles (see Arrhenius hypothesis).
An acid is a concoction animal groups that gives protons or hydrogen particles and additionally acknowledges electrons. Most acids contain a hydrogen molecule reinforced that can discharge (separate) to yield a cation and an anion in water. The higher the convergence of hydrogen particles created by an acid, the higher its acidity and the lower the pH of the solution.
An acid is a concoction animal groups that gives protons or hydrogen particles and additionally acknowledges electrons. Most acids contain a hydrogen molecule reinforced that can discharge (separate) to yield a cation and an anion in water. The higher the convergence of hydrogen particles created by an acid, the higher its acidity and the lower the pH of the solution.
The word acid originates from the Latin words acidus or acere, which signify "harsh," since one of the attributes of acids in water is an acrid taste (e.g., vinegar or lemon juice).
This table offers a review of the key properties of acids contrasted and bases.
Rundown of Acid and Base Properties :-
Property Acid Base
pH less than 7 greater than 7
taste sour bitter or lathery (e.gheati pop)
(e.g.vinegar)
scent burning sensation often no smell (special case is alkali)
surface sticky slippery
reactivity reacts with metals reacts with a few fats and oils
to deliver hydrogen gas
 Acid Examples :-
Acid Examples :-Arrhenius, Brønsted-Lowry, and Lewis Acids :-
There are various methods for characterizing acids. An individual alluding to "an acid" is typically alluding to an Arrhenius or Brønsted-Lowry acid. A Lewis acid is commonly called a "Lewis acid." The purpose behind the varying definitions is that these various acids do exclude a similar arrangement of atoms:
Arrhenius Acid: By this definition, an acid is a substance that builds the convergence of hydronium particles (H3O+) when added to water. You may likewise think about expanding the convergence of hydrogen particle (H+) as another option.
Brønsted-Lowry Acid: By this definition, an acid is a material equipped for going about as a proton benefactor. This is a less prohibitive definition since solvents other than water are not barred. Basically, any intensify that can be deprotonated is a Brønsted-Lowry acid, including normal acids, in addition to amines, and liquor. This is the most generally utilized definition of an acid.
Lewis Acid: A Lewis acid is an intensify that can acknowledge an electron pair to shape a covalent bond. By this definition, a few aggravates that don't contain hydrogen qualify as acids, including aluminum trichloride and boron trifluoride.
These are instances of kinds of acids and explicit acids:
Arrhenius acid
Monoprotic acid
Lewis acid
Hydrochloric acid
Sulfuric acid
Hydrofluoric acid
Acidic acid
Stomach acid (which contains hydrochloric acid)
Vinegar (which contains acidic acid)
Citrus extract (found in citrus natural products)
Solid and Weak Acids :-
Acids might be distinguished as either solid or feeble dependent on how totally they separate into their particles in water. A solid acid, for example, hydrochloric acid, totally separates into its particles in water. A feeble acid just somewhat separates into its particles, so the solution contains water, particles, and the acid (e.g., acidic acid).




 वे पौधों के गैर-प्रकाश संश्लेषक ऊतकों में पाए जाते हैं। वे प्रोटीन, लिपिड और स्टार्च की क्षमता के लिए उपयोग किए जाते हैं।
वे पौधों के गैर-प्रकाश संश्लेषक ऊतकों में पाए जाते हैं। वे प्रोटीन, लिपिड और स्टार्च की क्षमता के लिए उपयोग किए जाते हैं।